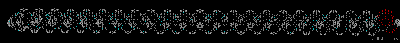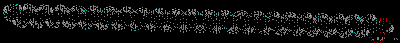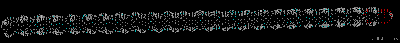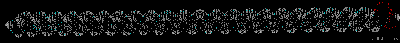|
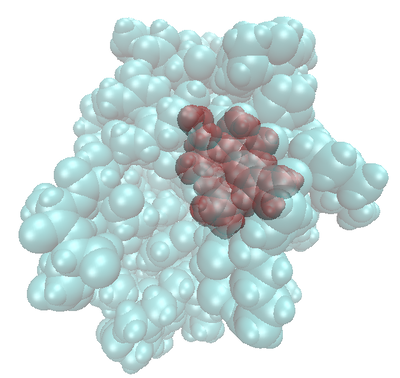
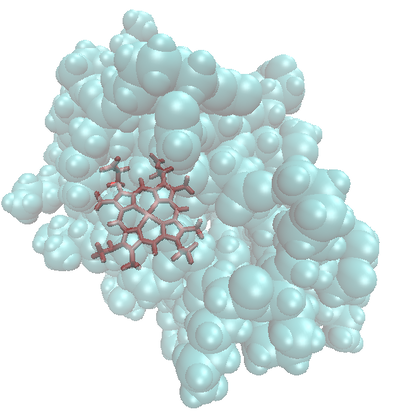
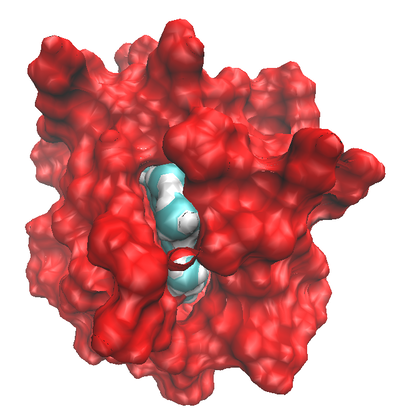
Montse Last week was a slow week. I have been learning new ways to code and visualize molecules using VMD, but am still working with some of the details in order to post some interesting pictures. Below are some preliminary examples (figures 1-3). Jo and I have discussed using other liquids to prepare the tea extract to try to get new colors. Examples of liquids discussed were nail polish remover (acetone and ethyl acetate), rubbing alcohol (isopropanol) and drinking alcohol (ethanol). In her last blog, Jo posted some new pictures that appeared more colored. I am intrigued and would like to know if any of those were obtained using these liquids (solvents). I have been thinking about the cyanotypes, and have a few ideas that might be interesting to try when preparing them. I will start my own experiments to try to reproduce the formation of mold on the tea. If mold appears (with the help of my colleague), we will try to identify what type of mold it is. This week I will also begin an art course that specializes in drawing plants. I am looking forward to drawing the tea plant from our lab. I will send the package to Jo tomorrow. Crystal structure of cytochrome c 1LC1 Jo
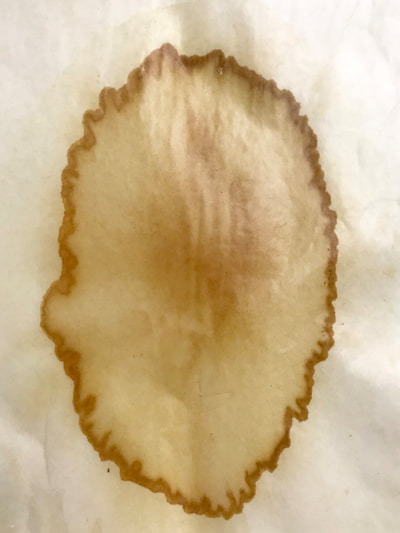
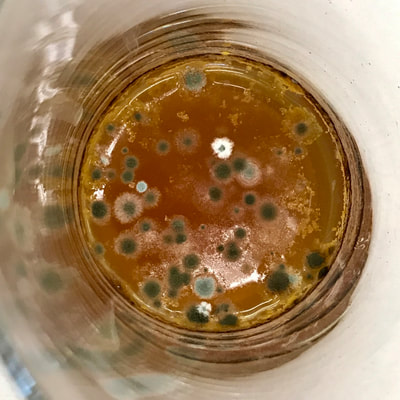
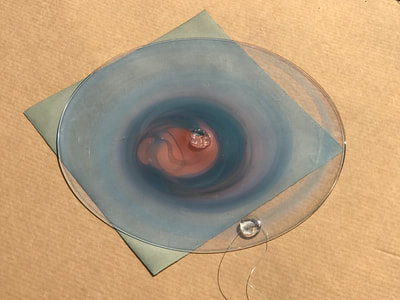
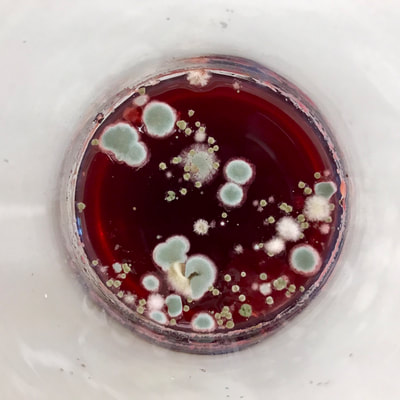
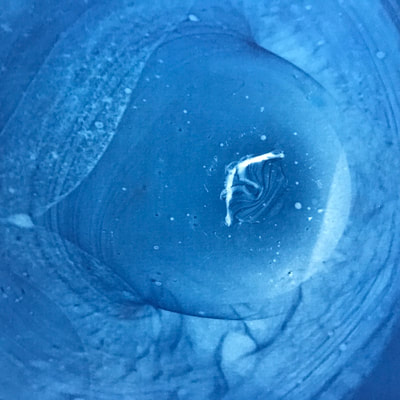
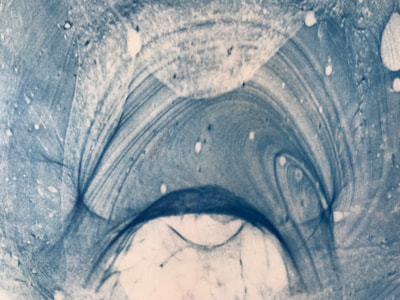
This past week has been a busy one with packing up studio work as I end a one month Artists Residency Fellowship at the Anderson Center in Red Wing, MN. I am on a flight back to New York City in a few hours so my blog will be more abbreviated than in past weeks. During the last seven days, I had a bit of time to continue some of the aspects of the collaboration as discussed last week. To continue the ongoing experiment with staining and molds, as per Montse’s and my conversation concerning the coloration of black and green tea due to processing, I only used those two types of teas to brew, stain and wax. This time, instead of selecting earlier inexpensive paper like newsprint I used the Chinese 1 ply paper, Dan Xuan (and also one piece of 2 ply Xu-jade Yuan) given to me by another Artist Resident who is from Quzhou, China. This paper allowed a bit more flow and subsequent detail in the ensuing stains from the brewed tea. However, after waxing the dried post-stained paper and watching the detail be less visible due to the wax, I might just stain the paper which is naturally transparent. I also was able to generate more mold with each of these two teas and wondered how the two new molds might compare what I processed with the original 7 teas. I also wondered how we might determine whether the mold that ensued in the steeped tea was from the walls, the atmosphere or perhaps even glass residue. As suggested by Montse, to promote the stain, I used rubbing alcohol (or perhaps next time will try acetone, both of which lack organic mode) as I tested color, mold and flow on small scraps of papers. Images of my experiments with stains, both with teas and with Cyanotype coating residue is included in the posted images. When back in my University studio next week, I would like to try some of the innovative approaches to Cyanotype chemistry suggested by Montse, such as combining ferric cyanide and tea (which as she mentioned might replace the hydrogen peroxide or the ferric ammonium sulfate). We also discussed how iron in blood might react, as in the tea, to the iron in the Cyanotype chemistry. As mentioned last week, we will focus on the properties of various teas and methods of testing/seeing. I continue to be interested in the visual and also scientific perspective on the stains with which I have been experimenting, using various teas and also papers for best/consistent results. I look forward to the teas and materials Montse is sending to me this week and will forward her my test results early next week once I unpack.
0 Comments
Montse In blog entry #2, Jo posted a series of cyanotypes that were simultaneously very good examples of beautiful art and chemistry. Cyanotypes are: “Photochemical blueprinting (also known as cyanotype process, from the Greek kyanos-blue) is one of the historically oldest photographic techniques that produce intensively blue pictures. Today it is classified as the member of a family of alternative photographic processes” quote taken from link 1. The chemistry of cyanotypes occurs in three main steps. The first one occurs when ferric ammonium citrate and potassium ferricyanide are combined and exposed to light. Light triggers the oxidation of citrate into carbon dioxide and the reduction of the iron (III) to iron (II). At this point the iron (II) and the ferricyanide form a solid known as Prussian Blue. Prussian Blue has a very unique blue color that occurs via intervalence charge transfer (IVCT) when electrons are transferred between iron (III) and iron (II). For details see link 1. The process involved in the preparation of cyanotypes is very similar to the research that we do in my group. The similarity occurs when an iron (III) containing protein (cytochrome c) is mixed with chemicals found in tea. This causes the reduction of the protein to iron(II), which, in turn changes the color of the protein from orange to pink. Due to this similarity, I was thinking that Jo could develop different types of cyanotypes using tea as the oxidizing agent for the iron. In blog entry #2, Jo posted a series of very amazing pictures of tea that grew mold on it. Neither Jo, nor I, expected to see mold growth. The fact that mold grew, made me question: Where is the mold coming from? What type of mold is capable of growing in tea extracts? What type of chemicals are produced after the tea is exposed to air for several days? There have been a few articles that aim to answer these questions,1-4 however many of the details remain unknown. Because of this, I hope to establish collaboration with a colleague that will help to shed some light on some of these questions. Links and references: Link 1 https://www.chemistryandlight.eu/theory/cyanotype-process/. References 1.Li, X.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W.; Ding, X., Determination for major chemical contaminants in tea (Camellia sinensis) matrices: a review. Food research international 2013, 53 (2), 649-658. 2.Londoño, V. A. G.; Reynoso, C. M.; Resnik, S. L., Polycyclic aromatic hydrocarbons (PAHs) survey on tea (Camellia sinensis) commercialized in Argentina. Food Control 2015, 50, 31-37. 3.Kim, Y.; Goodner, K. L.; Park, J.-D.; Choi, J.; Talcott, S. T., Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chemistry 2011, 129 (4), 1331-1342. 4.Chen, Y.; Xu, J.; Yu, M.; Chen, X.; Shi, J., Lead contamination in different varieties of tea plant (Camellia sinensis L.) and factors affecting lead bioavailability. Journal of the Science of Food and Agriculture 2010, 90 (9), 1501-1507. Jo
Montse and I had a long and fruitful conversation about the next step in our collaboration with its focus on the properties of various teas and methods of testing/seeing. I continue to be interested in the visual and also scientific perspective on the stains with which I have been experimenting, using various teas and also papers for best/consistent results. Following are the notes from our exchange: Things we discussed from our posts:
Exchanges being sent:
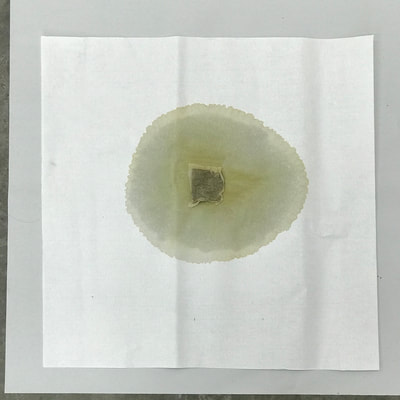
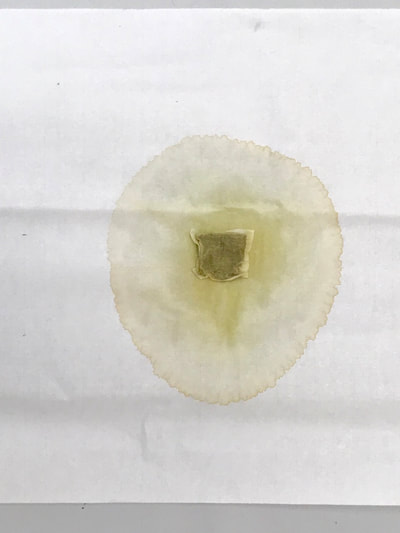
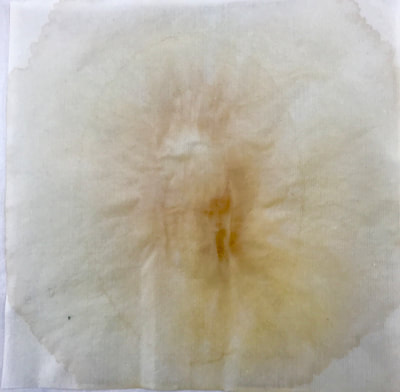
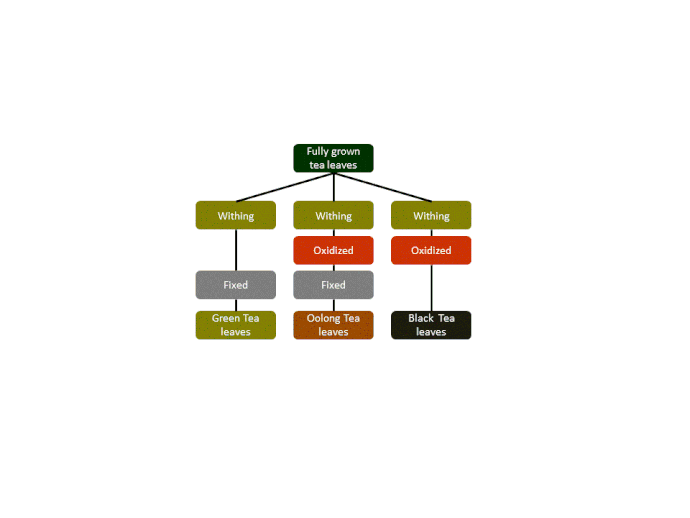
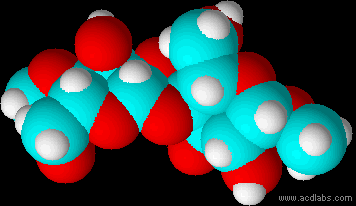
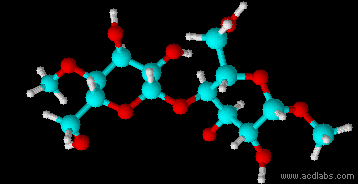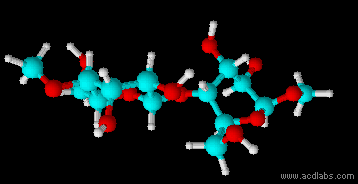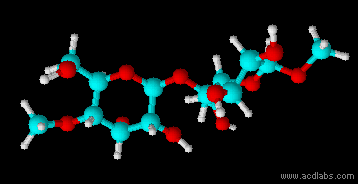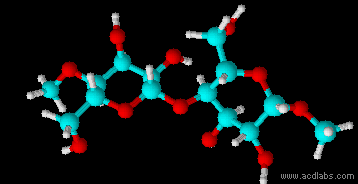
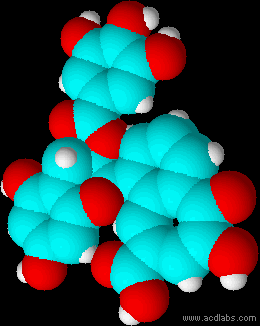
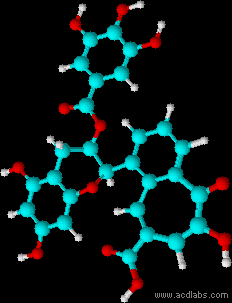
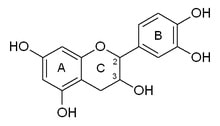
Montse Communications with Jo have been very stimulating. Unlike Jo, this is the first time I have had a serious collaboration with an artist. It has been good to learn that we have a lot of common interests, but the way we look at things is very different. I was very interested and fascinated with Jo’s experimentation with tea. Her approach was very systematic, just like a scientist setting up an experiment. However, the way she physically set up the space was intriguing and visually pleasing; it was clear that she takes into consideration the arrangement of her set up according to the space. She used seven different teas that contained black tea leaves or green tea leaves (Figure 1. Tea processing). When I look at the way I approach my experiments, I look at things at a micro and atomic level, (see link 2 and 3 for reference in scale). I look at the molecules present in the tea. For example, black tea leaves are darker in color because the chemicals present in the leaves are allowed to be oxidized. Additionally, when my group studies tea components, we are interested in the chemicals that are consumed by humans, such as epicatechin and epigallocatechin gallate EGCG (Figures 2-4), not the chemicals in the “residual bag” which usually goes into the trash. But instead, Jo used the tea bag to leave an imprint on the paper (made of cellulose, a polysaccharide composed of glucose repeating units (Figure 5-7). This made me think about the residual solid and what chemicals might be present in that solid. The transfer of chemicals from a solid media to paper using a liquid carrier is called paper chromatography, and we use a variety of liquids including water, alcohol and acetone as carriers as well. This process is very similar to Jo’s setup; therefore, it might be interesting if Jo were to use rubbing alcohol or acetone (different chemical properties) as a different medium to release other types of molecules into the paper (different molecules with different colors such as epitheaflavic acid 3’monogallate (Figure 8-10) and theflavic acid 3’ monogallate)1-3. Another topic that Jo used was systematic pattering. In organic chemistry, systematic pattering is also used to categorize chemicals according to their properties or structure. In my research this is very important because we look at how the changes in the structure affect the chemical properties of flavonoids (I will share a picture in the future, because we are writing a paper about this). After transferring the tea color into the paper, Jo added beeswax (Figures 11-14 show two chemicals that are commonly found in beeswax) to increase transparency. This was interesting to me, and is an area that I would want to further explore with Jo, and possibly do some measurements and experiments in my lab in the future. Links, figures and references: The atoms in all molecule figures are color coded: Carbon atoms (blue), Oxygen atoms (red) and Hydrogen atoms (white). Link 1 https://www.compoundchem.com/2014/02/01/polyphenols-antioxidants-the-chemistry-of-tea/ Link 2 Scale reference. https://www.khanacademy.org/science/cosmology-and-astronomy/universe-scale-topic/scale-earth-galaxy-tutorial/v/scale-of-the-small Link 3 Scale reference. https://www.youtube.com/watch?reload=9&v=HiN6Ag5-DrU References 1. Degenhardt, A.; Engelhardt, U. H.; Wendt, A.-S.; Winterhalter, P., Isolation of black tea pigments using high-speed countercurrent chromatography and studies on properties of black tea polymers. Journal of agricultural and food chemistry 2000, 48 (11), 5200-5205. 2. Kim, Y.; Goodner, K. L.; Park, J.-D.; Choi, J.; Talcott, S. T., Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chemistry 2011, 129 (4), 1331-1342. 3. TANAKA, T.; KOUNO, I., Oxidation of tea catechins: chemical structures and reaction mechanism. Food Science and Technology Research 2003, 9 (2), 128-133. Jo
As mentioned in my last blog entry for Bridge (evidence of a first, very enthusiastic foray into the blogdom world), I continue to be inspired by the premise of Monste’s work with teas, toxins and catechins. In that initial blog text, I noted key words that arose during the September 5th Skype conversation such as color, stains, molecular structures, sequential patterning, data and its codification, microscope slides and light. Using those words as framework, I have spent the last two weeks, through intuitive, object/process-based approaches, setting up and responding to “experiments”. Materials used have varied from the evolving residue of 7 steeped tea bags (from our shared kitchen cabinet), various papers, heated beeswax and Cyanotype photograms using glass objects from a local on-site glassmaker. My language is visual, so the following singular images and exploratory “pop-up” installations sited in various architectural structures at the Anderson Center complex are a chronological record of what has transpired. It is a journal of an analytical approach that is of my own making. What is arising through this process is the conceptual/metaphoric weight of the word “stain”. In our second Skype interview, I will be interested in discussing how my direction might inter-relate to Montse’s focus. I am curious about the many different levels on which this type of virtual collaboration might work. What I do find different from other collaborations is that in this one we are not sharing physical spaces, the deepening aspect of visual, non-verbal clues as we walk through those places (my studio/her laboratory) and the energy of one to one personal engagement. As a side note, I also have been following the Science Friday Podcast, a recent one was “When Plants Sense Danger, They Cry Out with Calcium”... metaphors abound! Montse What do I find exciting about this collaboration? I have always been intrigued by the processes and motives that lead artists to create art. I personally believe that there is a lot of common ground between art and science and I would be very excited to explore that common ground. Additionally, this collaboration will allow for the creation of novel strategies to educate students that are interested in art and science. I am very excited about working with Jo, I believe that we have many overlapping interests which in my opinion will translate into a very rewarding collaboration. One of the topics that we would like to explore is tea. Jo
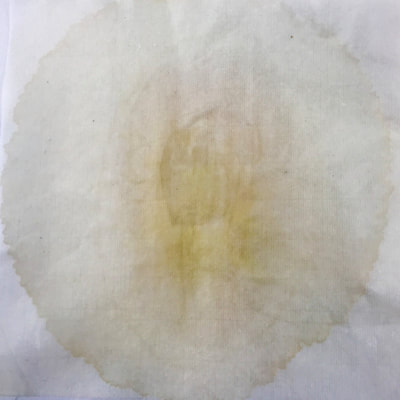
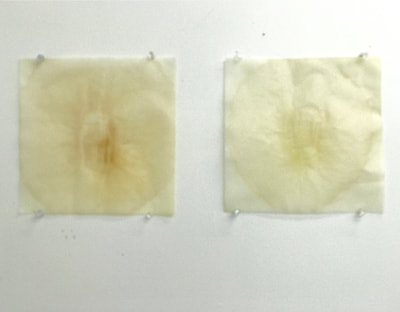
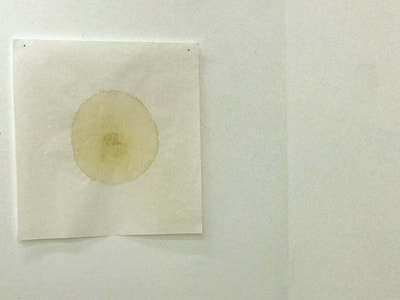
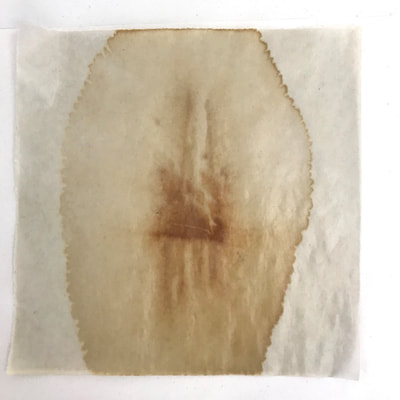
Much of my visual work over the last twenty year has been influenced by collaborations and the inherent organic processes and innovative directions that can evolve through collective engagement. Working with artists in other disciplines as well as with architects, musicians, composers, philosophers, physicists, environmental scientists and poets on site-specific exhibitions, public art commissions and collaborative projects has helped to provide in each circumstance a broader context and more expansive understanding of our shared foci. Recent projects have explored environmental issues and the relationship of language and image and so, with a growing interest in working more extensively with scientists, I was delighted to have been chosen for the Bridge residency and to have been paired with biochemist, Montse Rabago-Smith. During our first Skype interview with Julia, Montse and I were asked to describe ourselves as well as some of our research, our varying processes and also what methods we might use to articulate our findings. I found it refreshing to be encouraged not to focus on what “product” comes out of this 4 month interaction but merely to see how and where the dialogue leads us. During the Skype conversation, I listened to and noted the words we used to describe physical objects which were important to either Montse and/or to me, as well as the way we discussed their situational properties, such as - tea, anti-toxins, catechins, color, stains, molecular structures, sequential patterning, data and its codification (ie. diagrams, graphs, nano-imaging, direct observation, etc.), slides, light, etc.. Although we will be discussing possible directions for this residency in the next Skype interview in a few weeks, I decided to use as creative prompts during these first two weeks, a few of the words that we had spoken to each other. As an artist, I many times learn through tactile responses and direct experiences and right now, while I am on sabbatical and a Fellow at an artist residency, I have more extended time to creatively explore ideas using a range of materials in architectural space. In past projects, I have used varied combinations of glass, waxed surfaces, found objects and paper to investigate the way we perceive objects and images through our senses. These predominately translucent materials function as both a physical framework and symbolic membrane with implications of scale and the integration of architecture as pivotal components. With that premise of earlier work in mind, I decided to focus on and experiment with 7 different teas that are kept in the kitchen of the residency’s manor house. For each of the teas, I researched the correct steeping time to enhance their anti-toxin properties as well as augment their flavor. After steeping the tea bags in identical containers that held a pre- measured amount of hot water, each bag was placed on a 9”x 9” sheet of paper which I had pretested for maximum absorption and translucency. Once the teabags and papers were dry to the touch, I removed the tea from the paper and ironed each piece of paper to flatten before submerging it in a mixture of white and raw beeswax to better promote transparency. Later this coming week, I will install the waxed papers on to the windows of a greenhouse located on the residency property, calling attention to the windows’ slide-like quality and providing by the works’ siting, a direct link between plant, light, color and systematic patterning. The following images are labeled and in chronological order and provide a visual documentation of what I have been exploring on a mostly intuitive, material -based level. Interestingly enough, the final 3 images are of resident artist Pan Yu showing me how to make matcha, a green tea noted for its medicinal properties. We had been discussing this tea right before the Skype conversation. |