|
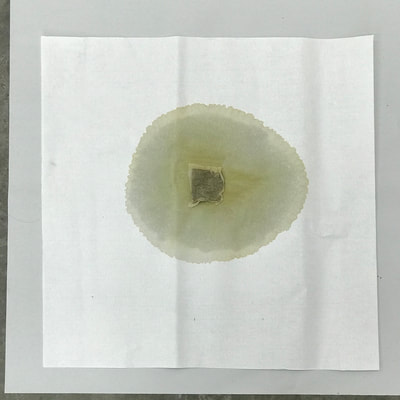
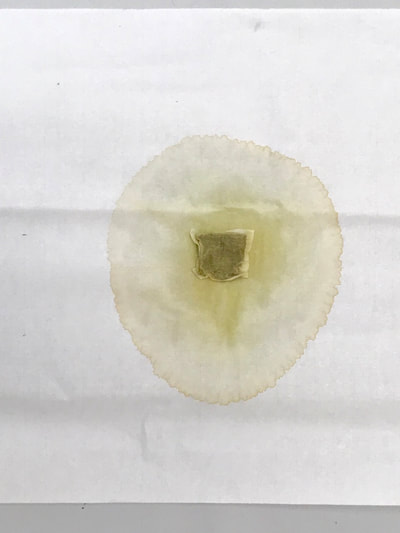
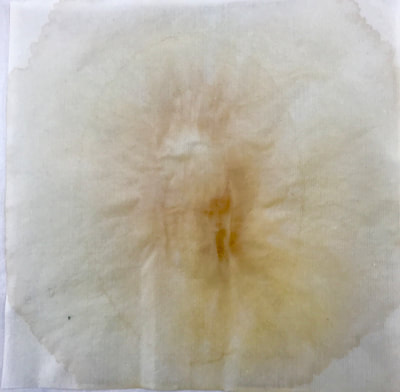
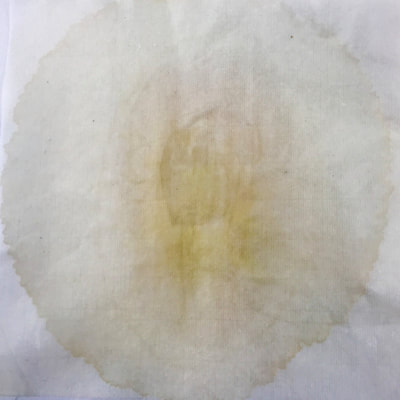
Montse In blog entry #2, Jo posted a series of cyanotypes that were simultaneously very good examples of beautiful art and chemistry. Cyanotypes are: “Photochemical blueprinting (also known as cyanotype process, from the Greek kyanos-blue) is one of the historically oldest photographic techniques that produce intensively blue pictures. Today it is classified as the member of a family of alternative photographic processes” quote taken from link 1. The chemistry of cyanotypes occurs in three main steps. The first one occurs when ferric ammonium citrate and potassium ferricyanide are combined and exposed to light. Light triggers the oxidation of citrate into carbon dioxide and the reduction of the iron (III) to iron (II). At this point the iron (II) and the ferricyanide form a solid known as Prussian Blue. Prussian Blue has a very unique blue color that occurs via intervalence charge transfer (IVCT) when electrons are transferred between iron (III) and iron (II). For details see link 1. The process involved in the preparation of cyanotypes is very similar to the research that we do in my group. The similarity occurs when an iron (III) containing protein (cytochrome c) is mixed with chemicals found in tea. This causes the reduction of the protein to iron(II), which, in turn changes the color of the protein from orange to pink. Due to this similarity, I was thinking that Jo could develop different types of cyanotypes using tea as the oxidizing agent for the iron. In blog entry #2, Jo posted a series of very amazing pictures of tea that grew mold on it. Neither Jo, nor I, expected to see mold growth. The fact that mold grew, made me question: Where is the mold coming from? What type of mold is capable of growing in tea extracts? What type of chemicals are produced after the tea is exposed to air for several days? There have been a few articles that aim to answer these questions,1-4 however many of the details remain unknown. Because of this, I hope to establish collaboration with a colleague that will help to shed some light on some of these questions. Links and references: Link 1 https://www.chemistryandlight.eu/theory/cyanotype-process/. References 1.Li, X.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W.; Ding, X., Determination for major chemical contaminants in tea (Camellia sinensis) matrices: a review. Food research international 2013, 53 (2), 649-658. 2.Londoño, V. A. G.; Reynoso, C. M.; Resnik, S. L., Polycyclic aromatic hydrocarbons (PAHs) survey on tea (Camellia sinensis) commercialized in Argentina. Food Control 2015, 50, 31-37. 3.Kim, Y.; Goodner, K. L.; Park, J.-D.; Choi, J.; Talcott, S. T., Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chemistry 2011, 129 (4), 1331-1342. 4.Chen, Y.; Xu, J.; Yu, M.; Chen, X.; Shi, J., Lead contamination in different varieties of tea plant (Camellia sinensis L.) and factors affecting lead bioavailability. Journal of the Science of Food and Agriculture 2010, 90 (9), 1501-1507. Jo
Montse and I had a long and fruitful conversation about the next step in our collaboration with its focus on the properties of various teas and methods of testing/seeing. I continue to be interested in the visual and also scientific perspective on the stains with which I have been experimenting, using various teas and also papers for best/consistent results. Following are the notes from our exchange: Things we discussed from our posts:
Exchanges being sent:
0 Comments
Leave a Reply. |